You must have a prescriber number to prescribe medicines. There are additional rules when you prescribe HSD, including the hospital you’re affiliated with. Read more about getting a provider and prescriber number.
HSDs are for the treatment of complex medical conditions that require ongoing specialised medical supervision. The HSD program is part of the PBS.
An authority approval is required from us for most HSD listings and increased quantities and repeats. Read more about Section 100 - Highly Specialised Drugs program on the PBS website.
Funded HSDs include Complex Authority Required Highly Specialised Drugs.
Restrictions apply to certain pharmacies that can dispense and claim PBS HSDs.
You can prescribe HSDs if you are both:
- a hospital-based prescriber or general practitioner
- an accredited prescriber of that medication.
You don’t have to be affiliated with a public or private hospital. The medications you can prescribe under the Community Access (CA) arrangements are:
- HIV ART for initial and maintenance therapy
- hepatitis B items for initial and maintenance therapy
- clozapine for maintenance therapy only
- opioid dependence treatment medicines.
Initial therapy with clozapine is under the HSD program and not part of HSD CA arrangements. Initial therapy must be started by an appropriate specialist who has an affiliation with a public or private hospital.
If you’re writing prescriptions for the PBS-listed quantity and repeats, you don’t need to contact us for authority approval. You can use the Authority-required (STREAMLINED) number listed on the PBS website if the use of the item meets the requirements of the listing.
If you’re prescribing increased quantities, repeats or both, you need to apply for a PBS authority.
Same day prescribing isn’t allowed for these medications.
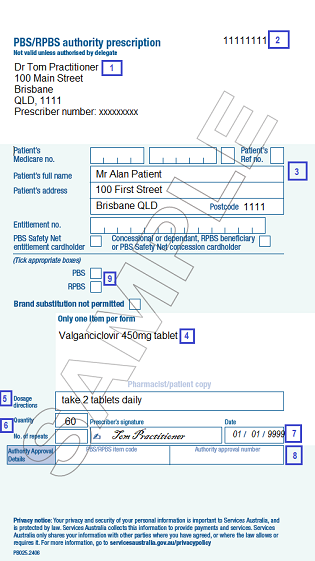
Include the following information on all HSD private hospital prescriptions:
- your name, practice address and prescriber number
- your patient’s name, address and card details
- authority-required item name, form and strength
- dose and instructions for use
- quantity and number of repeats
- your signature and the date the prescription was written
- authority approval number
- if the prescription is to be supplied under the PBS or Repatriation Pharmaceutical Benefits Scheme (RPBS)
- authority prescription number.
An authority prescription number is used by the pharmacy as a reference when dispensing. This number is generated by the prescribing software for computer generated prescriptions. The exact location of this number on the prescription may vary.
The active ingredient must be included on computer-generated prescriptions. Find out when active ingredient prescribing applies on the Department of Health, Disability and Ageing website.
Quote the hospital provider number when applying for an authority approval online or over the phone.
The hospital provider number must be written on the prescription for written applications. The hospital provider number isn’t required for HSD Community Access (CA) items.
Hospital prescriptions must include the hospital name and hospital provider number. Medication charts used in public hospitals must meet the same requirements as a hospital prescription.
Include the following information on all HSD public hospital prescriptions:
- hospital name, address, phone number, hospital provider number and the prescriber number and provider number.
- authority prescription number.
- your patient’s name, address and card details.
- authority required item name, form and strength.
- dose and instruction for use.
- quantity and number of repeats.
- your name, signature and the date the prescription was written.
- authority approval number.
- if the prescription is to be supplied under the PBS or Repatriation Pharmaceutical Benefits Scheme (RPBS).
The active ingredient must be included on computer generated prescriptions. Find out when active ingredient prescribing applies on the Department of Health, Disability and Ageing website.

Hospital prescriptions must include the hospital name and hospital provider number. Medication charts used in public hospitals must meet the same requirements as a hospital prescription.
You can prescribe HSD if you’re both:
- a hospital-based prescriber or general practitioner
- an accredited prescriber of that medication.
You don’t have to be affiliated with a public or private hospital. The medications you can prescribe under the arrangements are:
- HIV ART for initial and maintenance therapy
- hepatitis B items for initial and maintenance therapy
- clozapine for maintenance therapy only
- opioid dependence treatment medicines.
Initial therapy with clozapine is under the HSD program and not part of HSD Community Access (CA) arrangements. Initial therapy must be started by an appropriate specialist who has an affiliation with a public or private hospital.
If you’re prescribing increased quantities, repeats or both, you need to apply for a PBS authority from us.
Same day prescribing isn’t allowed for these medications.
Adequate and auditable systems should be in place so prescribing is in accordance with PBS restriction criteria.
The prescribing and supply of HSDs is monitored, and patients’ records may be audited with the agreement of the hospital.
Keep prescriptions and internal medication paperwork for a minimum of 2 years from the date of dispensing for audit purposes.